Standardizing microbiome therapeutics: challenges and innovations in GMP manufacturing and regulatory approval

Introduction
Over the past decade, the human microbiome has evolved from a promising scientific curiosity into a legitimate therapeutic frontier. Live Biotherapeutic Products (LBPs); whether derived from donor material or engineered microbial consortia; are advancing through late-stage clinical trials for a range of indications, from recurrent Clostridioides difficile infection (CDI) to graft-versus-host disease and metabolic disorders [1, 2, 3].
Despite this momentum, the path from bench to bedside remains complex. LBPs raise novel challenges in standardization, manufacturing, and regulatory classification. Ensuring safety, reproducibility, and scalability in this emerging space requires harmonized efforts across scientific, industrial, and regulatory domains [4].
Regulatory bottlenecks for LBPs
LBPs are composed of living microorganisms designed to confer clinical benefit. This differentiates them from conventional small molecules and biologics. Regulatory agencies face challenges such as:
- Identity and consistency: How can efficacy be maintained in products driven by dynamic microbial communities?
- Safety assessment: Absence of pathogens, antibiotic resistance genes, or unintended metabolites must be ensured.
- Clinical trials: Demonstrating causality and reproducibility is challenging due to biological complexity.
The EMA has categorized LBPs under frameworks similar to Advanced Therapy Medicinal Products (ATMPs), whereas the FDA regulates them as biologics [5, 6]. Both require Good Manufacturing Practice (GMP) compliance, but approaches remain fragmented.
The evolution of pooled microbial consortia manufacturing
Traditional Fecal Microbiota Transplantation (FMT) relies on minimally processed donor material. In contrast, new LBPs use pooled microbial consortia to enhance therapeutic reproducibility. Pooling strategies aim to:
- Increase microbial diversity
- Reduce batch-to-batch variability
- Improve reproducibility across patient populations
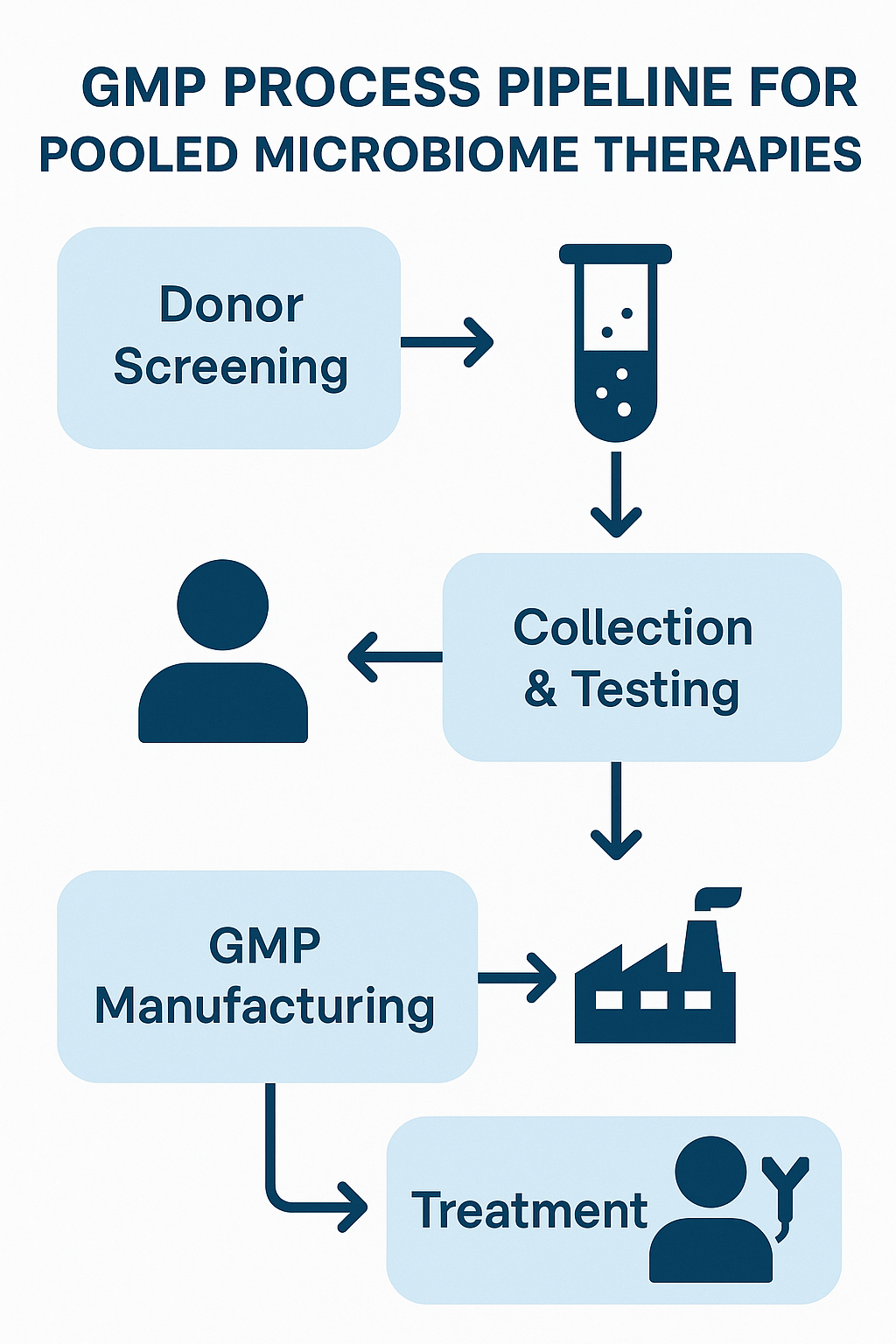
Pooled‐consortia manufacturing leaders include:
- MaaT Pharma’s Microbiome Ecosystem Therapies (METs), each batch pooled from multiple rigorously screened healthy donors under GMP to standardize taxonomic profiles and functional richness [7].
- Mikrobiomik’s MBK-01, a lyophilized, full‐spectrum intestinal microbiota therapy formulated for both oral and enema administration at pharmaceutical‐grade purity [8].
- Seres Therapeutics’ SER-109, a purified Firmicutes‐spore consortium that reduced recurrent C. difficile infections by 30.2% in Phase III studies [9].
- Finch Therapeutics’ CP-101, an orally administered blend derived from select healthy donors, shown in placebo‐controlled trials to prevent recurrent C. difficile infection [10].
- Rebiotix (Ferring)’s RBX2660, a rectally delivered, pooled‐microbiota suspension with positive preliminary Phase III data and Breakthrough Therapy Designation for CDI prevention [11].
- Vedanta Biosciences’ VE303, a defined eight‐strain oral consortium built from cultured isolates rather than raw fecal matter, advancing toward prevention of recurrent C. difficile infection [12].
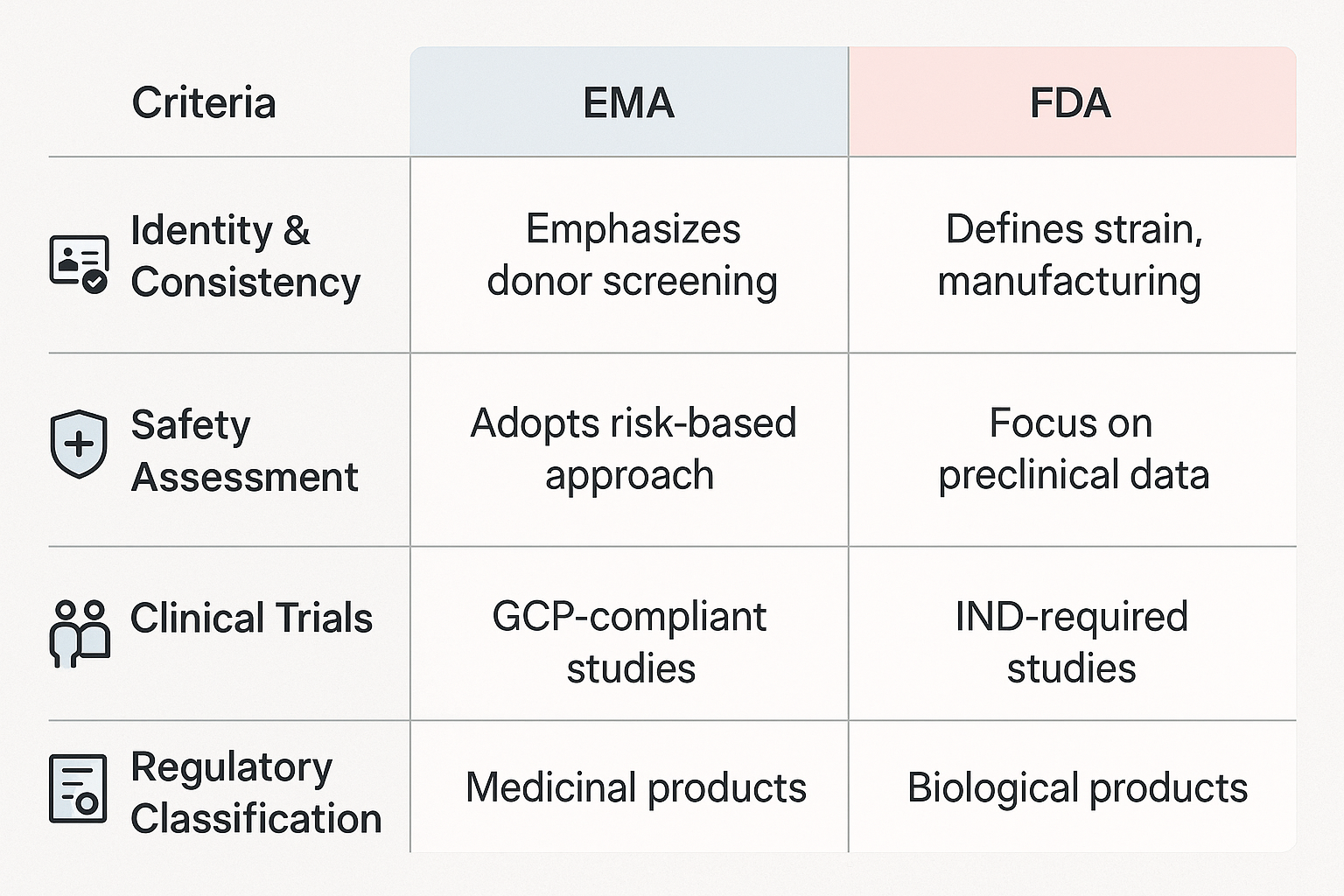
EMA and FDA perspectives
Both agencies acknowledge the promise of LBPs, but their priorities diverge:
- EMA emphasizes donor screening, genetic stability, validated GMP protocols, and post-market surveillance.
- FDA historically imposed clinical holds but is gradually adapting and allowing pivotal trials to proceed.
The FDA focuses more on preclinical characterization, while the EMA prioritizes real-world safety data. These regulatory differences introduce cost and timeline complexity for global developers [4].
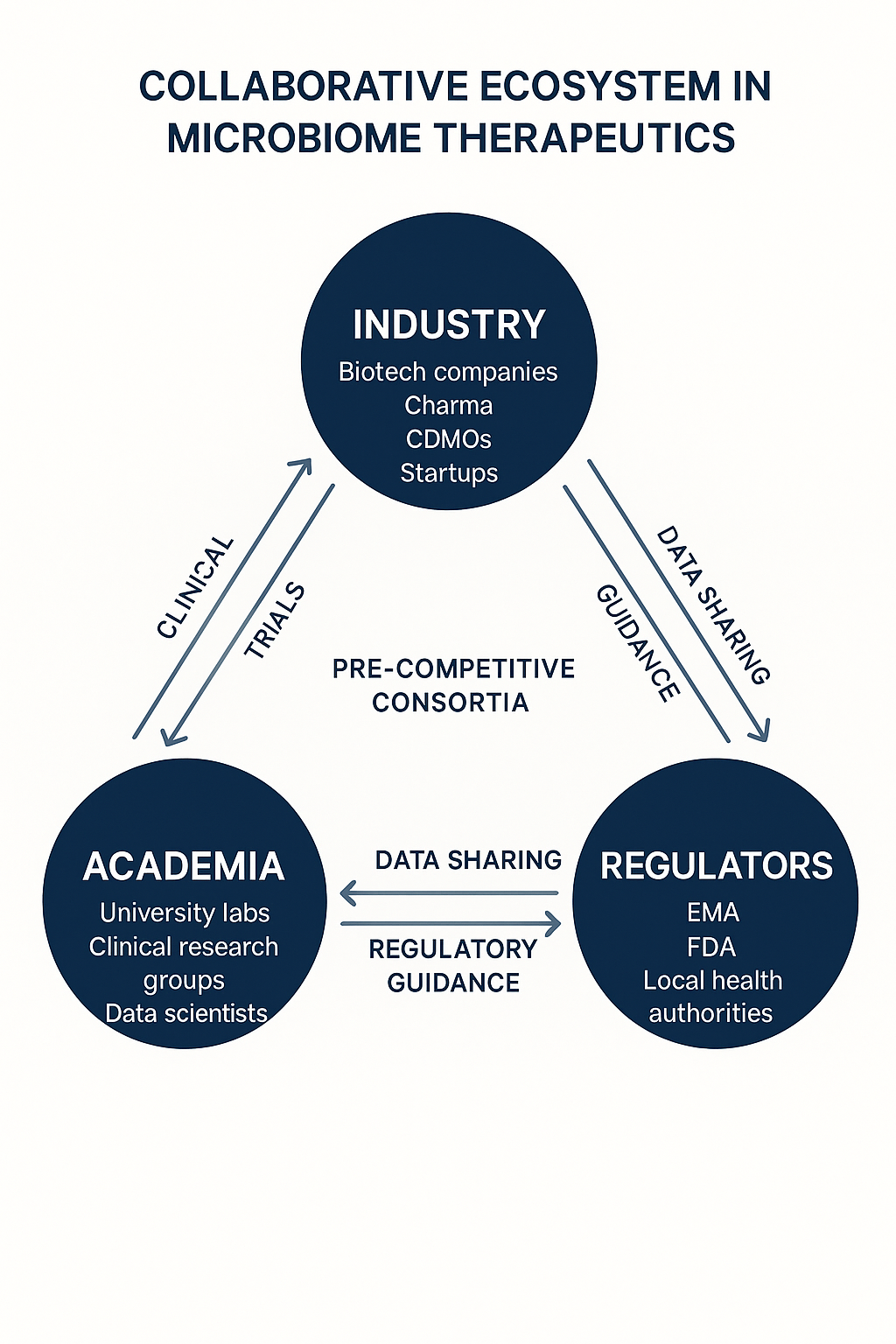
The path ahead: standardization without stagnation
To enable scalable, safe microbiome therapeutics, the following areas need prioritization:
- Defining microbial identity, potency, and purity metrics
- Harmonizing EMA and FDA regulatory guidance
- Integrating metagenomic/metabolomic analytics into GMP workflows
- Encouraging adaptive regulatory frameworks
Initiatives such as pre-competitive consortia, academic-industry working groups, and early dialogue with regulators will be key to shaping the future.
Conclusion
Microbiome therapeutics stand at an inflection point. Scientific validation is increasing, but scalable and compliant pathways remain under development. Companies like MaaT Pharma, Biose Industrie and Microbiomik are driving progress across manufacturing, quality, and clinical development.
Ultimately, standardization will require coordinated global efforts; not just from companies, but from regulatory bodies and the broader microbiome ecosystem. As the field matures, collaboration across innovation, manufacturing, and compliance will determine whether LBPs become a mainstream class of medicine or remain a niche innovation.
For further perspectives on the evolving microbiome field, explore our related articles. The Microbiome as a Critical Variable in Therapeutics delves into how microbiome research is transforming drug discovery, from spatial multi-omics to microbiome cartography, and why understanding microbial niches is key to unlocking new therapeutic strategies. Advancing Microbiome-Based Biomarkers in Inflammatory Bowel Disease (IBD) examines how metagenomics and metaproteomics are driving noninvasive diagnostics and more precise patient monitoring. Microbiome’s Role in Patient Care: Advances, Challenges, and Pharmaceutical Opportunities looks at how microbial balance shapes treatment outcomes, the development of novel therapies, and the regulatory and patient-centered models transforming healthcare. For a lighter angle, 🚇 Little Story of the Microbiome in Public Transportation Systems uncovers the hidden microbial ecosystems of subway networks, revealing how city life and microbiomes intertwine. Stay informed on the latest microbiome innovations, explore these related articles and join the conversation shaping the future of microbiome therapeutics!
References
[1] L Monday, G Tillotson, and T Chopra. “Microbiota-Based Live Biotherapeutic Products for Clostridioides difficile Infection”. In: Infection and Drug Resistance 17 (2024), pp. 123–134. https://doi.org/10.2147/IDR.S419243.
[2] T Lavoie, HJ Appaneal, and KL LaPlante. “Advancements in Novel Live Biotherapeutic Products for Clostridioides difficile Infection Prevention”. In: Clinical Infectious Diseases 76.5 (2023), pp. 890–898. https://doi.org/10.1093/cid/ciad639.
[3] BD Navalkele and T Chopra. “Clinical Application of Live Biotherapeutic Products in Infectious Diseases”. In: Frontiers in Microbiomes 3 (2024), pp. 101–112. https://doi.org/10.3389/frmbi.2024.1415083.
[4] D Barberio and Microbiome Therapeutics Innovation Group. “Navigating regulatory and analytical challenges in live biotherapeutic product development and manufacturing”. In: Frontiers in Microbiomes Volume 3 – 2024 (2024). issn: 2813-4338. https://doi.org/10.3389/frmbi.2024.1441290
[5] European Medicines Agency (EMA). Guideline on quality, non-clinical and clinical aspects of live biotherapeutic products. 2023. https://www.ema.europa.eu/en/quality-non-clinical-clinical-aspects-live-biotherapeutic-products-lbps.
[6] U.S. Food and Drug Administration (FDA). Live Biotherapeutic Products: Chemistry, Manufacturing, and Control (CMC) Information. 2022. https://www.fda.gov/vaccines-blood-biologics/cmc-guidance-documents.
[7] MaaT Pharma. Platform SKYE: Microbiome Ecosystem Therapies. 2023. https://www.maatpharma.com/platform-skye/.
[8] Mikrobiomik. MBK-01: Pharmaceutical-Grade Intestinal Microbiota Therapy. 2023. https://mikrobiomik.net/en/.
[9] Seres Therapeutics. Positive Phase III Results for SER-109. 2023. https://ir.serestherapeutics.com/news-releases/news-release-details/seres-therapeutics-announces-positive-topline-results-ser-109.
[10] Finch Therapeutics. CP-101 Prevents Recurrent C. difficile Infection in Placebo-Controlled Trial. 2020. https://www.finchtherapeutics.com/publications/cp101-an-investigational-orally-administered-microbiome-therapeutic-increases-intestinal-microbiome-diversity-and-prevents-recurrent-c-difficile-infection-results-from-a-randomized-placebo-contro/.
[11] Rebiotix (Ferring). Positive Preliminary Phase III Data for RBX2660. 2021. https://www.ferring.com/rebiotix-and-ferring-announce-worlds-first-with-positive-preliminary-pivotal-phase-3-data-for-investigational-microbiome-based-therapy-rbx2660/.
[12] Vedanta Biosciences. VE303: Defined Consortium for rCDI Prevention. 2024. https://www.vedantabio.com/pipeline-programs/ve303/.
