AI and ML in medical devices: Navigating innovation, regulation, and cybersecurity

Paris, June 5-6, 2025 – Lucid Analytics, in partnership with ExpertsMedTech, successfully delivered a two-day training session in Paris focused on “AI and Cybersecurity: the new challenges”. This event brought together industry leaders, regulatory experts, and innovators to explore the transformative potential of artificial intelligence and machine learning in healthcare, while addressing the critical challenges of regulation, data security, and cybersecurity.
A collaborative approach to medical device innovation
The training, led by Pierre Laye (Lucid Analytics) alongside renowned experts, provided a deep dive into the development, validation, and deployment of AI/ML-powered medical devices. Participants engaged in interactive discussions, case studies, and practical workshops, gaining actionable insights into:
- The fundamentals of AI/ML in healthcare: From statistical learning to deep learning, and their applications in diagnostics, patient assistance, and treatment optimization.
- Regulatory and ethical considerations: Navigating the European MDR (2017/745), GDPR compliance, and the balance between innovation and patient safety.
- Cybersecurity best practices: Securing connected medical devices in an era of increasing digital threats.
Key highlights
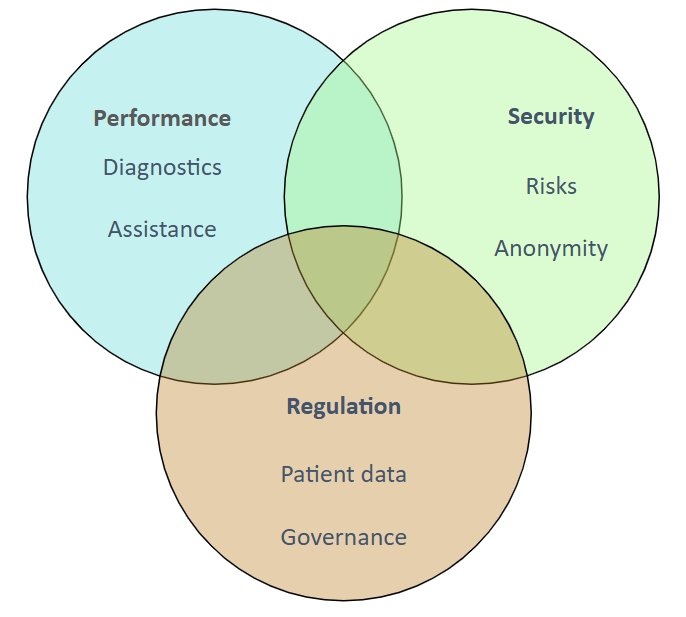
The intersection of performance, security, and regulation
The training emphasized the triple challenge facing medical device developers:
- Performance: Ensuring diagnostic accuracy and clinical efficacy.
- Security: Protecting sensitive patient data and device integrity.
Regulation: Aligning with evolving standards and certification processes.
As Pierre Laye noted, “Integrating AI into medical devices isn’t just about algorithms, it’s about building trust through transparency, robustness, and compliance.”
Expert perspectives
The event featured a lineup of distinguished speakers:
- Cyrille Michaud (MD101) offered a comprehensive overview of the current regulatory landscape, highlighting the technical and legal hurdles for AI-driven devices.
- Thomas Lommatzsch (AFNOR Group) shared the perspective of a standardization body, discussing how to adapt to dynamic regulatory frameworks.
- Alain Ribault (Kereval) provided real-world examples of cybersecurity implementation, stressing the importance of proactive risk management.
Hands-on learning: from theory to practice
Attendees explored a case study on AI-powered patient chatbots, illustrating the end-to-end process of:
- Needs assessment and data acquisition.
- Model development (e.g., supervised learning for response matching).
- Validation and deployment, including user testing and regulatory documentation.
A prototype demo using FastAPI and Docker showcased how to rapidly iterate and deploy secure, scalable solutions.
Industry networking and knowledge exchange
The session fostered vibrant discussions among participants, ranging from startups to established medtech firms, on topics such as:
- Data quality and bias mitigation in AI models.
- Edge vs. cloud computing for real-time medical applications.
- Collaborative strategies to accelerate innovation while ensuring compliance.
Why this matters for the MedTech industry
AI and ML are reshaping healthcare, enabling:
- Personalized treatments through predictive analytics.
- Enhanced diagnostics via image recognition and natural language processing.
- 24/7 patient support through intelligent chatbots and remote monitoring tools.
Yet, as Mathieu Lang (Lucid Analytics) remarked, “The true potential of AI in medicine lies not just in its capabilities, but in our ability to implement it responsibly.”
Looking ahead
Lucid Analytics remains committed to advancing safe, ethical, and high-performance AI solutions for the medtech sector. We extend our thanks to all participants, speakers, and partners for making this event a success. Stay tuned for future training opportunities and collaborations!
Missed the event? Contact us to learn about upcoming sessions or tailored training for your team.
